Description
Principle
The natural alkalinity of cement paste in concrete results in a protective oxide coating on steel reinforcement that prevents the steel from rusting. When carbon dioxide (CO2) in the air penetrates into concrete, it reacts with the calcium hydroxide (CaOH2) in the cement paste producing calcium carbonate (CaCO3). This reaction is called carbonation, and it causes the alkalinity of the paste to decrease, that is, the pH decreases below its normal value of about 13. When the pH drops below 9, the protective oxide coating is destroyed and, in the presence of moisture and oxygen, the steel will corrode. Thus measurement of the depth of carbonation is an essential step for corrosion evaluation of a reinforced concrete structure.
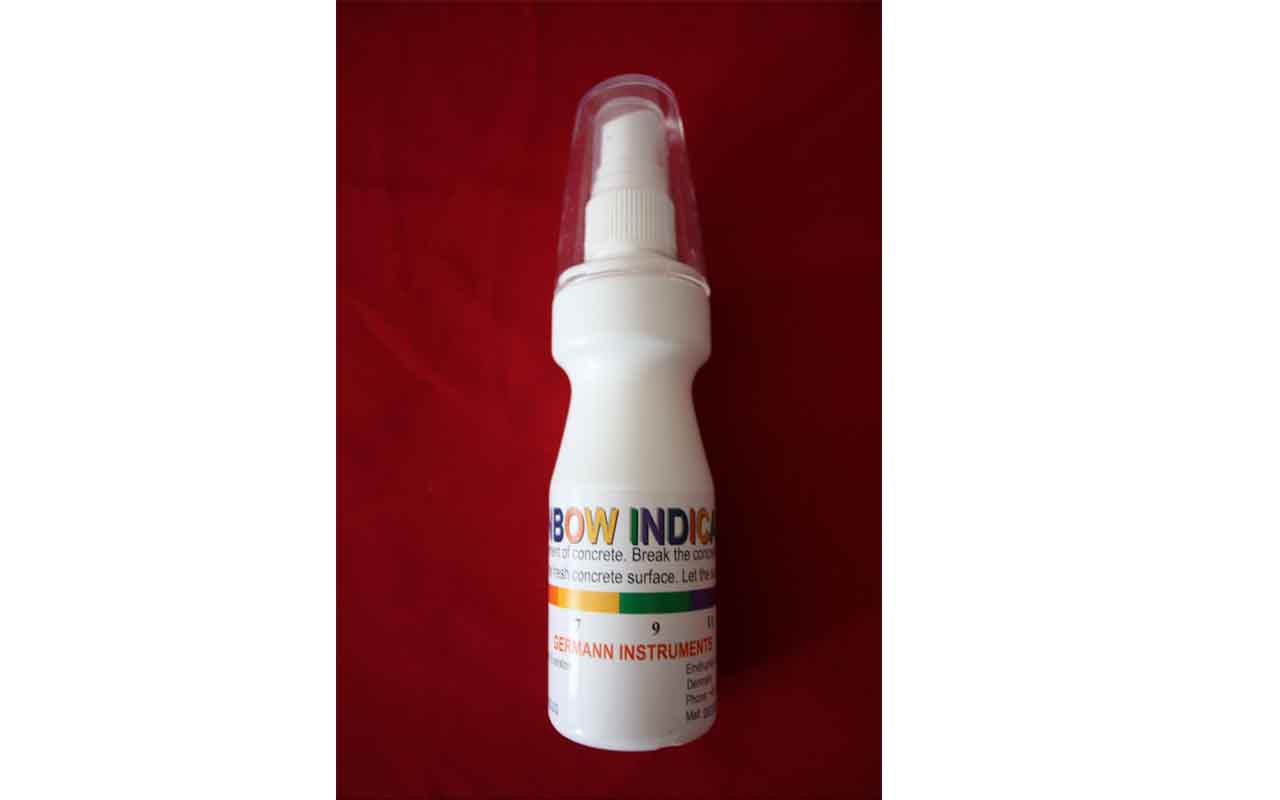
To measure the pH of the cement paste, a freshly broken piece of concrete or a newly cut core is sprayed with the indicator, and allowed to dry. The approximate pH of the paste is indicated by colors as illustrated below.
Deep Purple Indicator
 Rainbow Indicator
Rainbow Indicator

Accuracy
The carbonation front measured with the Deep Purple Indicator represents where the cement paste has a pH within the range of 8.5 to 9.5 as shown above.
The results of the Rainbow Indicator were correlated with the depth of carbonation determined by petrographic thin section analysis for a wide range of concretes with varying slump, with or without calcium chloride or fly-ash, different water-cement ratios, varying degrees of consolidation and different finishing methods. The results were published in:
Campbell, D.H., Sturm, R.D. and Kosmatka, S.H., “Detecting Carbonation,” Concrete Technology Today, Vol. 12, No. 1, March 1991, Portland Cement Association, USA
The results indicated that the depth of carbonation determined from thin section analysis correlated with the depth where the Rainbow Indicator indicated a green color or pH of 9 as shown above.
On normal concrete, the depth of the carbonation front can be determined with an accuracy of ± 10 % to ± 15 %.






Reviews
There are no reviews yet.